| Product No. | CH061 |
|---|---|
| CAS Reg. No. | 119478-55-6 |
| Alternate CAS Reg. No. | - |
| Offer | 100 mg |
119478-55-6
- Documentation
- Details
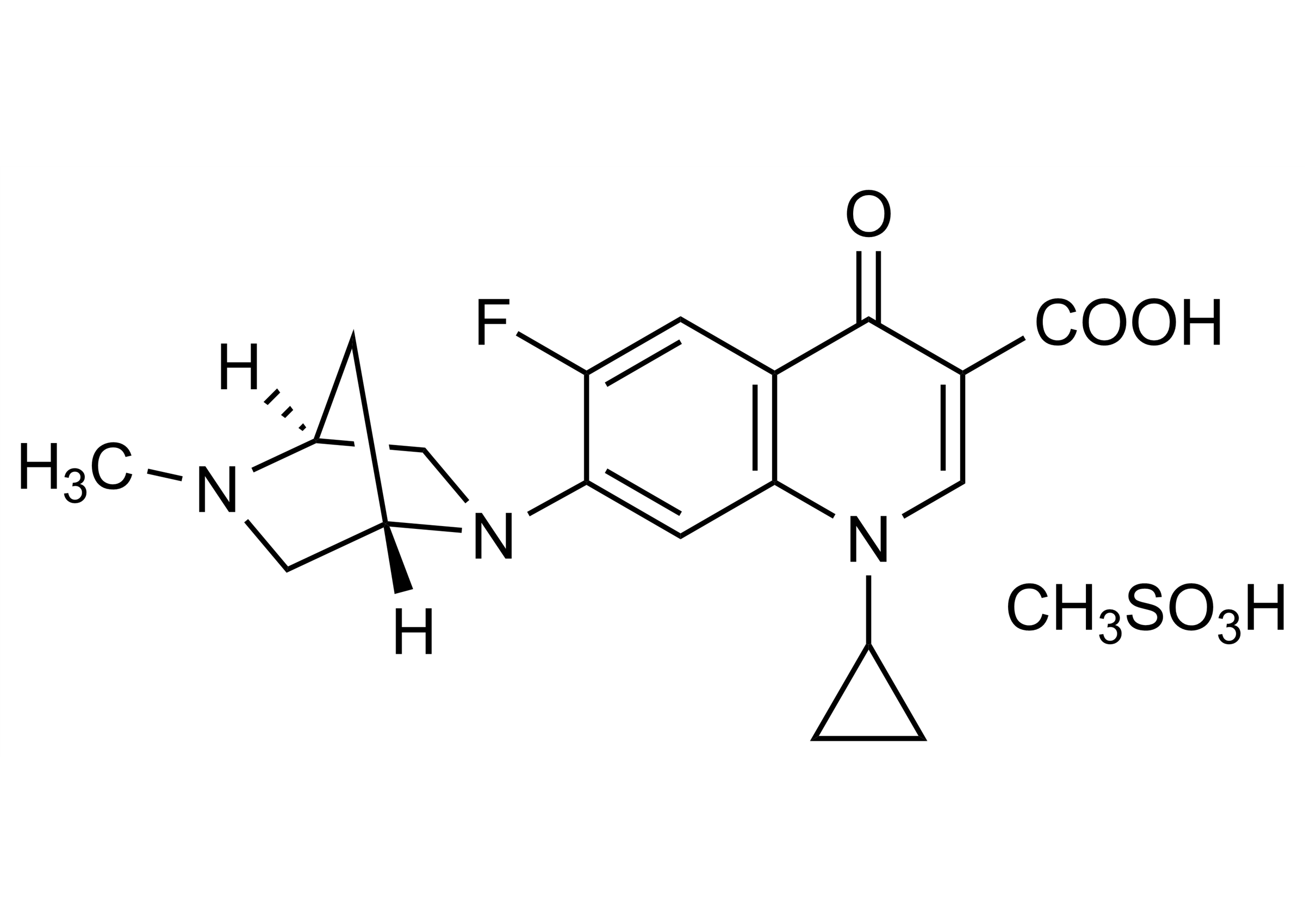
Chemical name
1-Cyclopropyl-6-fluoro-7-[(1S,4S)-5-methyl-2,5-diazabicyclo[2.2.1]hept-2-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid methanesulfonate
Description
Danofloxacin methanesulfonate (CAS 119478-55-6) is a high-quality fluoroquinolone reference standard produced and distributed by WITEGA Laboratorien Berlin-Adlershof GmbH. Use this reference standard to achieve confident LC-MS/MS and GC-MS quantification, robust calibration, and defensible confirmatory analysis. It supports traceability from preparation to reporting, which strengthens method validation in regulated workflows. Moreover, it offers consistent performance for multi-matrix residue testing and advanced research. Consequently, laboratories streamline assay development and reduce rework.
Designed for modern mass spectrometry, this reference standard enables accurate calibration curves, routine system suitability checks, and ongoing quality control. Additionally, it helps verify recovery and precision during matrix-matched validation. You can rely on the stable composition and detailed documentation to maintain traceability and compliance. Furthermore, Danofloxacin methanesulfonate supports confirmatory analysis criteria, ensuring clear, reproducible identification and quantification.
Typical applications include:
- Regulated laboratories focusing on veterinary drug residue control and surveillance
- Pharmaceutical research exploring fluoroquinolone metabolism and pharmacokinetics
- Residue control programs for food safety and environmental monitoring
- Metabolism studies in target species and in vitro systems
- Multi-residue method development across LC-MS/MS and GC-MS platforms
Key advantages:
- Traceable preparation with clear documentation for audits and data defensibility
- Lot-to-lot consistency that supports long-term method performance
- Optimized for calibration, matrix validation, and confirmatory testing
- Broad applicability across complex matrices and analytical workflows
Choose this reference standard from WITEGA Laboratorien Berlin-Adlershof GmbH to enhance accuracy and reproducibility in advanced quantification. Danofloxacin methanesulfonate (CAS 119478-55-6) fits seamlessly into targeted panels, method transfers, and routine QC, so teams can validate faster and report results with confidence.
Safety Data Sheet
You can download your Safety Data Sheet for CH061
For other languages please contact us: witega@witega.de
Additional information
| Chemical name | 1-Cyclopropyl-6-fluoro-7-[(1S,4S)-5-methyl-2,5-diazabicyclo[2.2.1]hept-2-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid methanesulfonate |
|---|---|
| Molecular Formula | C19H20FN3O3 x CH3SO3H |
| Molecular Weight | 453.49 g/mol |
| Isotopic purity | - |
| HPLC purity | 98.8 ± 0.7 % (283 nm) |
| Overall purity | 98.8 ± 0.7 % (HPLC) |
| Product Format | Neat |
| Delivery time | In stock |
| shelf life | 24 months |
| Storage | refrigerator, 2-8°C |
| Country of Origin | Germany |
| Product No. | CH061 |
| CAS Reg. No. | 119478-55-6 |
| Alternate CAS Reg. No. | – |
| Offer | 100 mg |